How to Know if You Popped an Artery
Open access peer-reviewed affiliate
Basics of Angiography for Peripheral Artery Disease
Submitted: September seventh, 2016 Reviewed: December 7th, 2016 Published: March 22nd, 2017
DOI: x.5772/67177
IntechOpen Downloads
three,823
Total Chapter Downloads on intechopen.com
Abstruse
Angiography has been historically used to paradigm the peripheral artery organisation and still remains the gilded standard for diagnostic and endovascular treatment. There is no standardized method for lower limb artery angiography. In this affiliate, the bones standard technique for angiography of peripheral artery is described from aortoiliac, femoropopliteal and beneath the genu arteries. To obtain a adept image, adequate contrast dose and image size must exist determined with the appropriate catheter. For puncture, echo-guided approach is becoming popular; each lab needs to have echo auto to minimize the vascular complication. In cases of renal dysfunction, CO2 angiography is suited. However, care must be taken to evangelize gas into arterial system and to know the merit and demerit of CO2 angiography.
Keywords
- peripheral artery
- peripheral artery illness
- contrast angiography
- echo-guided puncture
- CO2 angiography
*Address all correspondence to: smtyokoi@gmail.com
1. Angiography-suite for endovascular therapy of peripheral avenue disease (PAD)
i.1. Detector size
Loftier resolution, accurate imaging is the key to success in endovascular therapies. In contempo years, most machines provide fairly good images. An important point is the detector size of the angiography machine. Some physicians yet use a coronary lab for peripheral artery intervention, nonetheless, when because the vessel length and area, at least a xxx cm detector is needed. In Figure ane , two types of detectors are shown.

Figure 1.
Detector size: (A) 30 cm × 30 cm, (B) 20 cm × 20 cm. In peripheral avenue angiography, at least a 30 cm image size is needed. The twenty cm image size is besides pocket-sized for peripheral artery angiography.
In peripheral avenue angiography, the 30 cm system on the left (INNOVA 3100, xxx cm, GE healthcare, Uppsala, Sweden) ( Figure 1A ) is basically used while the 20 cm coronary organization (INNOVA IGS620, 20 cm, GE healthcare, Uppsala, Sweden) ( Figure 1B ) is too modest for peripheral avenue angiography. For example, the superficial femoral avenue (SFA) is the longest vessel and difficult to visualize in its entirety. In Figures 1 and 2 , two SFA short lesions are shown.
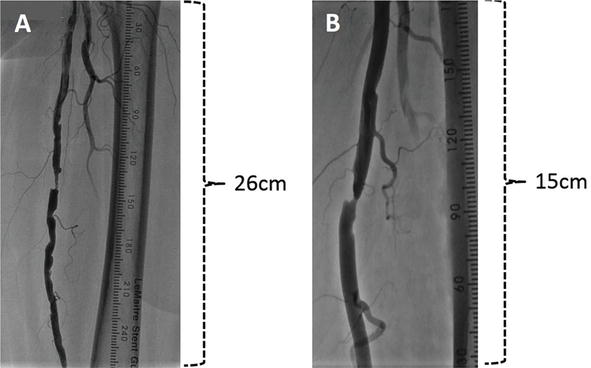
Figure ii.
Image field of xx cm and 30 cm detector in SFA. (A) In the xxx cm detector, about 26 cm of SFA is visualized. (B) In the xx cm detector, only xiii cm of SFA is seen.
In the 30 cm console, about 26 cm of the SFA tin exist visualized and intermediate stenosis around the culprit lesion ( Figure 2A ) can be discerned. On the other hand, the coronary detector could visualize simply thirteen cm of the SFA in xx cm mode ( Figure 2B ). In a coronary lab, to visualize the SFA or below the knee (BK) arteries, the table is panned but a skillful static image of the lesion is difficult to obtain.
1.2. Extra monitor
In an angiographic suite, operators ordinarily stand on the right side of the table. About labs use one monitor and all medical staffs rely on this one screen. In right limb angiography via the left femoral artery approach, the operator who is standing on the right side has difficulty manipulating the catheter. In this situation, i operator needs to stand up on the left side of the table to manipulate the catheter and hold the sheath. For this purpose, an actress-monitor should be installed ( Figure 3 ).

Figure 3.
Actress-monitor. In correct superficial artery (SFA) intervention, the master operator stands on the left side of the table watching the extra-monitor while the assisting operator watches the primal monitor. Without moving the central image monitor, the master operator tin can manipulate the catheter from the left side.
A typical right superficial artery (SFA) intervention is shown in Figure 3 . The main operator is standing on the left side of the table and watching the extra-monitor while the assisting operator keeps an eye on the key monitor. Without moving the central paradigm monitor, the master operator is able to perform the process. This extra-monitor is useful in the left brachial approach as well. It is a user-friendly way to intervene in the right femoropopliteal artery or cross-over approach for right below the knee arteries. In the left beneath the knee artery procedure via the cross-over approach, the C-arm is rotated to the left side. The cranial side operator may not see the central epitome. In this situation, the extra monitor can be placed on the left cranial side.
1.3. Injector
For most of the small vessels in selective angiography, hand injection of the contrast dye is adequate. However, for optimal opacification of high-flow blood vessels like the aorta, the use of a ability injector is mandatory. A constant and high volume of dye should be injected through an electronically calibrated power injector. At that place are 2 types of injectors: one is a conventional power injector and the other is an assisted device that introduces minor or big amounts of dye by an injector attached to the catheter tabular array. The dissimilarity volume is adjusted manually and so that even a small dose of dye tin can be injected. However, the infinite on the left side of the table is occupied past this assisted device. Thus, a conventional power injector mounted to the ceiling is preferable since it affords more space around the catheter tabular array. Furthermore, the distance allows a meaning reduction in radiation exposure during dye injection. With the assisted device, radiation exposure is difficult to prevent since the operator has to exist abreast the table during dye injection ( Figure four ).

Figure 4.
Ability injector mounted to the ceiling. The ceiling-mounted injector allows more space effectually the catheter tabular array.
1.4. Contrast dose
Contrast-related factors include the vascular admission site, injection time duration, injection rate, contrast volume and dye concentration. The cardinal factor is the injection rate. An increased charge per unit of injection can induce a greater extent of vascular opacification but the safety and full book of the contrast dose must exist carefully monitored. The dissimilarity volumes for opacification of the major arteries are shown in Tabular array i . These are the injection volumes mainly used in our catheter laboratory although the actual dissimilarity book depends on the patient's status, the catheter size, amount of dissimilarity and speed of injection. Therefore, the contrast dose should be individualized for each case.
| Location | Catheter | Injection rate (ml/s) | Total volume (ml) |
|---|---|---|---|
| Aortoiliac | 5Fr Pig tail | 14–sixteen | fifteen–25 |
| CFA-SFA-Pop A | iv–5Fr MP | 5–vii | 16–20 |
| Run: CFA-BTK | 4–5Fr MP | 4 | nine–12 |
| SFA | four–5Fr MP | 4–v | viii–ten |
| Run: BTK | 4Fr MP | 3–iv | 10–12 |
| BTK | 4Fr MP | 3–4 | v–seven |
| Below the ankle | 4Fr MP | 3–4 | 5–7 |
Table one.
Dissimilarity injection rate and injection book.
There is no universally agreed upon threshold in the degree of renal dysfunction beyond which intravascular iodinated contrast medium should not be administered. We use Visipaque 320 [2]. Contrast-induced nephropathy (CIN) is an infrequent agin reaction to iodinated contrast agents [3]. In endovascular procedures, particular complex procedures are associated with CIN and larger doses of contrast are considered a risk factor. Thus, every bit a precaution against CIN, the use of contrast media at the lowest dosage possible is advised. To minimize the dissimilarity dose, nosotros dilute Visipaque 320 by adding 30 cc of saline solution in a 100 cc bottle. The central factor is the injection rate which indicates the corporeality of dye per second. In our experience, ane/3rd diluted contrast does not subtract image quality.
1.5. Radiation condom
Angiography machines which employ fluoroscopy for endovascular work are equipped with pulsed fluoroscopy instead of continuous fluoroscopy and this, to a large extent, helps to reduce the radiations dose (3 radiation pulse mode). During this procedure, both the patient and md are exposed to a sure caste of radiation so that its dose needs to be minimized. Constant measurement of radiation doses in patients and personnel is vital. Above all, the shielding in the room is particularly of import. We use a suspended ceiling shield equally well as a floor installed shield ( Figure five ). During digital subtraction angiography (DSA) imaging, other comedical staffs are exterior the angiosuite. The main operator besides the patient is protected by a ceiling-mounted radiation shielding glass. After the process, radiation exposure levels must be routinely recorded and archived.

Figure 5.
Radiations shield. Operator uses the ceiling-mounted radiation shield and the assistant is backside the shield during contrast injection.
Advertizing
ii. Imaging techniques
2.i. Sheath
2.1.1. 4Fr sheath
The 4Fr sheath is mainly used for the antegrade femoral approach. For initial admission, a 4Fr sheath is placed from the common femoral artery (CFA) to the SFA. The reason is that an antegrade puncture is technically more demanding and if we fail to make the puncture, the sheath tin be withdrawn or repositioned. While keeping the 4Fr sheath in the profunda femoris artery (PFA), we can even identify an additional 4Fr sheath into the CFA. The long 4Fr sheath is for below the genu work. Notwithstanding, it is hands kinked and there may be an increased gamble of hematoma formation. In interventions beneath the knee joint arteries, near occlusion balloons accept the 4Fr sheath with the use of a 0.014 or 0.018 in. guidewires. And to minimize sheath size in the ipsilateral CFA approach, a 4Fr long sheath is platonic for patients with critical limb ischemia (CLI) ( Figure 6A ).

Figure 6.
Sheath. (A) 4Fr sheath, (B) 5Fr sheath, (C) 6Fr sheath.
2.1.2. 5Fr sheath
In advertizement hoc interventions, we accept standardized the 5Fr sheath for the initial retrograde CFA approach. When stent implantation is planned, nosotros start with a 6Fr sheath. Either a 4Fr or 5Fr pigtail catheter can be used for aortography. With a 4Fr pigtail catheter, the amount of dye is limited to around 10–xiii cc/s. To opacity the concluding aorta to both the iliac and common femoral arteries, the rate of injection should exist 15–20 cc/s and this flow rate can be achieved with at to the lowest degree a 5Fr pigtail catheter. Introducer sheaths are used for all angiography and endovascular procedures. The 5Fr 45 cm cantankerous-over sheath is used for either the retrograde or antegrade arroyo. In a contralateral SFA intervention, a 5Fr 45 cm crossover sheath is used. Notwithstanding, when stenting is performed, the sheath should be replaced with a 6Fr crossover sheath. In the antegrade approach for BK interventions, a 5Fr 45 cm crossover sheath gives more dorsum-up back up to intervene on the tibial arteries ( Figure 6B ).
2.1.3. 6Fr sheath
When an iliac artery stent is already planned, a 6Fr short sheath should exist placed in a retrograde manner. In a cross-over approach, a 6Fr 45 cm cross-over sheath is employed. The advantage of the 6Fr system is that the closure device tin be applied later on the procedure. In some medical centers, the antegrade 6Fr curt sheath is placed for SFA stenting. However, we do non routinely use the 6Fr sheath for antegrade piece of work ( Effigy 6C ) ( Table 2 ).
| Advantage | Disadvantage |
|---|---|
| Better wire control with brusque wire | Complication related to antegrade common femoral artery (CFA) puncture |
| Brusque distance to the lesion | Might miss proximal superficial femoral artery (SFA) lesion |
| Precise stent placement | Demand circumspection of proximal end of stent |
| Admission to below the genu arteries | Pinch of ischemic side later on procedure |
Table ii.
Advantage and disadvantage of ipsilateral antegrade approach.
2.two. Wires
2.2.ane. 0.035 in. wire
At that place are three types of tips for the 0.035 wire. We practise not use a regular J-tip Radifocus wire (Terumo, Tokyo, Japan) ( Figure 7A ). The initial wire is always a 1.five mm J-type Radifocus wire (Terumo, Tokyo, Japan) ( Figure 7B ). The tip of this wire has a 1.5 mm circular shape and is quite safety when the wire migrates into the small branches or other vessels. Once the guidewire crosses the lesion, we change to a regular 0.035 in. spring wire ( Figure 7C ). The Radifocus wire is glace and is difficult to go along in place while regular bound wires tend to stay in identify. Thus, for stability, the wire should be changed to a spring wire one time the lesion is crossed. These 3 types of 0.035 in. wires should always be at hand.

Figure 7.
0.035 in. guidewires. At that place are three types of tips for the 0.018 in. wire. We do not use regular a J-tip Radifocus wire (A). The initial wire is ever a i.v mm J-type Radifocus wire (B). Once used to cantankerous the lesion, information technology is exchanged to a 0.018 in. jump wire (C).
ii.2.2. 0.018, 0.014 in. wires
Basically, we practise not use the 0.018 in. wire as a regular wire. Chronic total occlusion (CTO), a 0.018 in. Treasure 12-g (Asahi Intec, Nagoya, Japan) wire is initially selected. It has a 12-g tip load and is best suited as a peripheral CTO wire. The Five 18 (Boston Scientific, Cambridge, MA, United states) wire has a strong main shaft with a soft tip and can be used for cross-over ballooning or stent implantation. There are many 0.014 in. wires and their purposes vary. For below the knee joint artery work, the 0.014 in. wire is the basic wire used.
2.3. Digital subtraction angiography (DSA) vs. digital angiography (DA)
Digital subtraction angiography (DSA) has long been the gilded standard for evaluation of atherosclerotic lesions in patients with PAD. Prototype quality has been further improved by replacing traditional image intensifiers with flat panel detectors so that regular digital angiography (DA) is at present replacing DSA. When considering the high radiation doses, non all cases need imaging past DSA. Above all, critical limb ischemia is difficult to manage and some patients have difficulty staying still during injection of the contrast dye. Thus, acceptable optimization with either DSA or DA should be employed to obtain accurate imaging of the diseased segments.
2.iii.1. Aortoiliac avenue
A typical DSA prototype of the iliac artery is shown in Figure viii . In the 30 cm image, we tin can encounter from the terminal aorta to both common femoral arteries ( Figure 8A ). In the 20 cm paradigm, a clearer view tin be seen ( Figure 8B ).
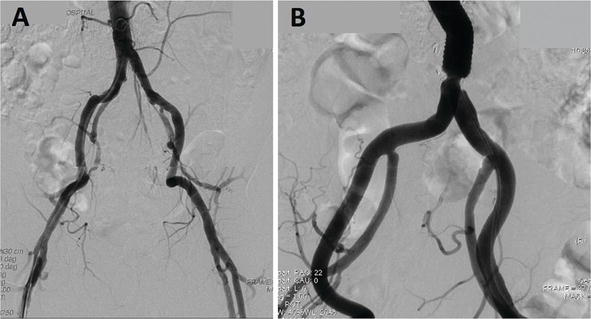
Figure 8.
Iliac artery angiography, 30 cm vs. 20 cm epitome. (A) xxx cm prototype, we could meet from the terminal aorta to both mutual femoral arteries. (B) 20 cm prototype, a clearer view is obtained.
In our routine, nosotros beginning take a thirty cm paradigm by DSA ( Figure 9A ). Next, we have a xx cm epitome by DA for the purpose of intervention ( Figure 9B ).

Effigy 9.
DSA vs. DA image of the iliac artery. (A) 30 cm image by DSA for diagnostic purposes. (B) xx cm image past DA for interventions. DSA, digital subtraction angiography; DA, digital angiography.
DA is more practical for stent implantation since it provides the groundwork image. In the aortoiliac artery segment, the epitome is hampered by bowel and gas movements. Aortoiliac artery angiography is basically taken by DSA, however, due to bowel and gas movements, the image is blurred ( Figure 10A ). In such a circumstance, nosotros alter to the DA paradigm ( Figure 10B ). In Figure 10 , a left common iliac aneurysm with distal stenosis tin can exist seen; the DSA image is blurred while the DA image conspicuously reveals stenosis.

Effigy 10.
DSA vs. DA epitome of the iliac avenue. (A) DSA image is blurred by bowel gas. (B) DA image shows clear paradigm of left mutual iliac aneurysm with distal stenosis. DSA, digital subtraction angiography; DA, digital angiography.
2.three.2. Femoropopliteal artery
The initial angiographic image is the ipsilateral angled view. Either DSA or DA can provide a reasonable paradigm ( Figure 11 ), although the DSA image ( Figure 11A ) is shown to be better than the DA image ( Figure 11B ). In the DA image, the groundwork is shown and tin exist used as reference ( Figure 11B ).

Effigy 11.
Proximal femoral artery. (A) DSA image for ipsilateral angled view of the left proximal femoral artery. (B) DA image shows the background and identifies bifurcation betoken.
A calcified lesion is often seen in the common femoral artery. In such cases, DSA provides a clearer view than the DA image ( Figure 12 ). DSA clearly shows the calcified lesion ( Figure 12A ) while, in contrast, the lesion could not be determined in the DA image due to low contrast ( Figure 12B ).

Figure 12.
Calcified mutual femoral artery. (A) DSA clearly shows calcified lesion. (B) DA image could not determine lesion due to depression contrast.
For the SFA, nosotros use either the DSA or DA image. For a calcified lesion, DSA is preferable ( Figure 13A ), just in near cases, DA provides a reasonably good image ( Figure 13B ).

Figure 13.
SFA angiography, DSA vs. DA. (A) DSA shows clearer image and branches are well seen. (B) DA gives reasonably good prototype.
In a SFA lesion, measurement of the lesion length is important to make up one's mind the interventional strategy and we prefer a DA epitome for the pre-interventional angiogram. The popliteal artery is located deep in the posterior fossa of the human knee joint. Surrounded by a bony structure, the popliteal artery is very difficult to visualize by DA. Basically, a DSA image is taken for the popliteal artery ( Figure 14A ). In Figure 14A , tight stenosis of the mid-popliteal artery is well visualized with rich collateral circulation. In the DA view, stenosis is well observed but virtually of the collateral vessels are non visualized ( Figure 14B ).
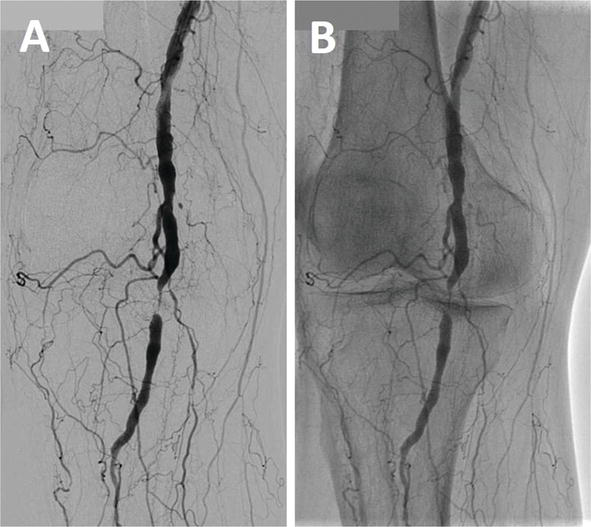
Effigy fourteen.
Popliteal artery angiography, DSA vs. DA. (A) Popliteal artery surrounded by bone and basically taken with DSA. (B) DA view shows well visualized stenosis merely near collateral vessels unclear.
two.3.3. Below the knee arteries
Diseases of below the genu arteries are closely associated with critical limb ischemia (CLI) and detailed anatomical information is required to plan intervention. Compared to other limb arteries, angiography remains the imaging method of option in most cases of CLI. How to take a adept image is the cornerstone of successful endovascular therapy. DSA is a must for imaging of below the articulatio genus arteries. In Figures ii – 10 , comparisons of the DSA and DA images of the left proximal tibial arteries are shown. In the DSA prototype, posterior tibial artery occlusion is well observed ( Figure 15A ). On the other mitt, the DA prototype failed to show the tibioperoneal trunk and occlusion of the posterior tibial avenue ( Effigy 15B ).

Figure 15.
Proximal below the knee angiography, DSA (A) vs. DA (B).
A similar instance of the left proximal below the genu artery is shown in Effigy 16 . In the DSA paradigm, three tibial arteries are shown with multiple stenosis ( Figure 16A ). In the DA image, precise diagnosis cannot be made ( Effigy 16B ).

Effigy xvi.
Mid-beneath the human knee angiography, DSA (A) vs. DA (B).
Assessment of the distal tibial arteries is vital in evaluating below the talocrural joint affliction. Continuation from the inductive tibial avenue to the dorsal avenue and the posterior tibial artery to the planted artery must be antiseptic. However, due to the bony structure, the DA prototype could not testify these distal tibial and below the ankle arteries ( Figure 17 ). In Effigy 17A , the planter avenue is non conspicuously visualized in the DSA prototype. In the DA prototype, nigh of the vessels remain un-visualized ( Figure 17B ).

Figure 17.
Beneath the talocrural joint angiography, DSA (A) vs. DA (B).
2.4. Basic angiography for PAD
2.4.one. Angiography from the terminal aorta to beneath the human knee artery
In an angiographic approach for PAD diagnosis, nosotros need to assess three segments of the lower limb avenue, that is, the aortoiliac, femoropopliteal and below the knee arteries. In Figure eighteen , the basic angiography is shown. First, angiography of the aortoiliac artery was taken ( Figure 18A ). The second angiography is an ipsilateral view of the proximal femoral artery ( Effigy 18B ). In the right leg, a 30° right anterior oblique (RAO) view was chosen to separate the proximal SFA and PFA. 3rd, angiography from the CFA to the distal below the ankle artery was taken past running the table ( Effigy 18C ). Subsequently observing these 3 angiograms, we could assess in which segment stenosis or apoplexy was located.

Figure xviii.
Basic lower limb artery angiography. (A) Aortoiliac artery angiography. (B) Proximal femoral artery by a 30° right anterior oblique view. (C) From the right common femoral artery to the distal below the ankle artery.
A typical claudication with SFA disease is shown in Figure 19 . Aortoiliac artery angiography showed no significant stenosis ( Figure 19A ). In the proximal femoral artery, there was no stenosis in the SFA and PFA ( Figure 19B ). Left limb angiography showed focal stenosis in the mid-SFA while the left inductive tibial artery was non visualized ( Figure 19C ). By using DA, left SFA angiography was taken and revealed focal tight stenosis in the mid-SFA ( Effigy 19D ). This DA image was used as reference in interventional work ( Effigy 19D ).

Effigy xix.
A typical claudication with SFA disease (A) Aortoiliac artery angiography showed no significant stenosis. (B) In the proximal femoral artery, at that place was nostenosis in the SFA and PFA. C: Left limb angiography showed focal stenosis in themid-SFA. (D) LeftSFA angiography revealed focal tight stenosis in the mid-SFA.
Effigy 20 shows isolated below the knee avenue disease. From the iliac to femoropopliteal artery level, no atherosclerotic changes could be observed ( Figure 20A and 20B). A lesion is located in the right below the human knee arteries. Below the human knee arteries showed a stenotic lesion of the anterior tibial artery, and the posterior tibial avenue and peroneal avenue are occluded ( Effigy 20C ). This type of lesion, that is, "isolated below the genu artery disease" is often found in patients with critical limb ischemia.

Effigy 20.
Basic lower limb avenue angiography. Isolated below the knee artery illness. (A) Aortoiliac avenue showed no disease. (B) Right femoropopliteal artery showed no disease. (C) Right below the knee arteries showed a stenotic lesion of the anterior tibial artery, and the posterior tibial artery and peroneal avenue are occluded.
2.iv.ii. Magnification of images
The epitome size has two purposes: one is to see the whole vessel, for example, in the aortoiliac artery, visualization from the terminal aorta to both the right and left CFA ( Figure 21A ). The other is to better intervene on the target lesion utilizing appropriate magnification of the epitome size ( Figure 21B ). For wiring to this lesion, a 20 cm magnified prototype was taken and successful wiring was carried out using a 0.014 in. wire ( Figure 21B ).

Effigy 21.
Iliac artery angiography, 30 cm (A) vs. 20 cm prototype (B).
In Figure 22 , right SFA stent restenosis was visualized with a thirty cm image ( Figure 22A ). Moreover, using the 20 cm magnified mode, stent restenosis was well observed ( Figure 22B ).

Figure 22.
SFA angiography, 30 cm (A) vs. 20 cm paradigm (B).
In below the human knee arteries, the whole image shows which vessels are diseased ( Effigy 23A ). However, this running paradigm does not give detailed information on the three tibial arteries. In the xxx cm image, the three proximal tibial arteries are well observed, and the peroneal and posterior tibial arteries are diffusely diseased ( Figure 23B ). The farther magnified twenty cm image revealed that at that place is tight stenosis at the ostium of the correct anterior tibial artery ( Figure 23C ).

Figure 23.
Below the genu angiography, 30 cm (A), 20 cm (B) and sixteen cm images (C).
2.four.3. Pre- and postinterventional image
Basically, all interventional work requires two images to be taken, that is, pre- and postintervention. These two images reveal the angiographic changes pre and post process. In Figure 24A , the femorofemoral bypass was occluded and a long full occlusion of the correct iliac artery is seen. After successful recanalization and stenting, angiography of the exact same iliac artery was taken ( Figure 24B ).

Effigy 24.
A case of right iliac artery occlusion. Pre (A) and post iliac avenue angiography (B).
In Figures 2 – 20 , typical left SFA apoplexy was seen ( Effigy 25A ). Later balloon angioplasty, dissection and incomplete dilatation were observed ( Effigy 25B ). Post stent angiography showed splendid dilatation of the left SFA lesion ( Effigy 25C ). During the procedure, the table was frequently moved and oftentimes, mail angiographic images were not taken in the dissimilar positions, giving a false impression of the postinterventional prototype.

Figure 25.
A case of SFA occlusion. Pre (A) and post SFA angiography (B).
Advert
3. Repeat-guided puncture femoral artery puncture
The common femoral artery (CFA) remains the most widely accepted site for endovascular avenue access. Vascular access site-related complications are a major crusade of periprocedural morbidity amidst patients undergoing percutaneous endovascular intervention. In detail, patients with PAD may be more than likely to have atherosclerosis affecting the CFA. Ultrasound guidance is an emerging tendency for all percutaneous procedures and its employ for femoral artery puncture has decreased vascular complications and improved first-pass success rates [4–6].
3.i. Retrograde common femoral artery puncture
The CFA is the principal access site for angiography and interventional procedures. Among the various puncture sites, the retrograde CFA puncture is the most unremarkably employed and the basis of arterial punctures. We take described a safe and echo-guided technique for avoiding femoral admission site complications.
3.1.1. Puncture point
The inferior border and upper border of the femoral head should be realized by fluoroscopy ( Figure 26A ). Later checking the maximum arterial pulse ( Figure 26B ), Xylocaine is given 1 cm below the middle of the femoral caput ( Figure 26C ).

Figure 26.
Puncture bespeak of common femoral artery. (A) Realizing the inferior border and upper edge of the femoral head by fluoroscopy. (B) Marker eye of femoral head. (C) Xylocaine to be given i–2 cm below.
3.ane.2. Preparation
A sheath and two types of wires were prepared. One time a puncture is performed, the wire should be prepare to be inserted and if at that place is resistance, change to a unlike kind of wire is brash ( Effigy 27 ).

Figure 27.
Preparation of sheath and two types of wire. Set up sheath and two blazon wires shut to puncture site. Once puncture completed, insert wire and if resistance encountered, modify to different shape wire.
3.1.3. Echo scanning
For an echo-guided puncture (NEMIO MS, Toshiba, Tochigi, Nihon), first, echo scanning was carried out from the upper CFA to proximal SFA ( Figure 28 ). We could identify where the bifurcation is located. Either a long axis ( Figure 28A ) or short axis tin be obtained ( Effigy 28B and C ). A scan is basically made by a short-centrality view. The ideal puncture site of the CFA can then exist located ( Figure 28B ) and the bifurcation site tin can be identified ( Figure 28C ).

Figure 28.
Color Doppler scanning from CFA to SFA and PFA. (A) Long-centrality view of CFA and SFA. (B) Short-axis view of CFA. (C) Short-axis view of SFA and PFA. Using color Doppler, scan from upper CFA to SFA and PFA. Locate the ideal puncture site of CFA and place the bifurcation point. CFA, common femoral artery; SFA, superficial femoral artery; PFA, profunda femoral artery.
An repeat image is all-time seen from the upper common femoral avenue to the distal external iliac artery. When full reliance is on echo guidance, the puncture site locates college than the middle femoral head. To avoid besides low or high punctures, rechecking the puncture site by fluoroscopy is advised ( Figure 26A ).
three.ane.4. Puncture
Arterial access was obtained with an 18-Yard needle (Cook Medical, Bloomington, Indiana) using the modified Seldinger technique. The needle was inserted at an angle of about 45° from the skin at a level just below the heart of the femoral head. In viewing the short axis, the aim should exist for the elevation of the vessel. During flash backs of blood, a gentle wire insertion must be fabricated. When resistance is felt, change from a straight wire to circular shaped wire is advised ( Figure 29 ). When the plaque in the CFA is found, a normal CFA puncture site should be located. In Figure 29A , the long-centrality view showed the plaque in the CFA.

Figure 29.
Puncture. (A) Echo guidance. (B) 18G needle puncture. In viewing short axis, aim for peak of the vessel. During flashback of blood, gentle wire insertion should be made. If resistance encountered, change direct wire to round shaped wire.
In this situation, a plaque gratuitous zone within the CFA should be located ( Figure 30B – D ).

Effigy 30.
Presence of CFA plaque. (A) Long-centrality view of CFA and SFA. Annotation CFA plaque. (B) Plaque costless site of CFA in brusk axis. (C) Presence of plaque. Should not exist punctured. (D) SFA and PFA level. When finding plaque in the CFA (A), should wait for normal CFA puncture site. Must find plaque complimentary zone within CFA. CFA, common femoral artery; SFA, superficial femoral artery; PFA, profunda femoral artery.
In Figure 31 , a puncture was made at the site of CFA disease and the wire went into false lumen, resulting in the total occlusion of the CFA.

Figure 31.
Puncture of common femoral artery plaque. (A) Puncture into CFA plaque. Creates imitation lumen. (B) TIMI 0 period. (C) Dissection of iliac artery. Without knowledge of CFA disease, puncture was made. Wire went into false lumen and ended up in total occlusion of CFA.
iii.two. Antegrade common femoral artery puncture
For treatment of femoropopliteal artery disease, the standard arroyo has been to access the contralateral mutual femoral artery (CFA). However, an ipsilateral, antegrade CFA approach has sure advantages. Compared to the contralateral approach, access to the lesion distance is curt which in turn improves the responsiveness of the wire handling used to perform the intervention. In other clinical situations such as post aorto-bi-femoral surgical featherbed, deployment of iliac kissing stents, post stent grafting and for aortoiliac occlusive disease, an antegrade approach is the method of selection to accomplish the lesion. The advantages and disadvantages are shown in Table 2 .
The CFA is approximately 4–5 cm in length and arises from the external iliac artery (Eia) as information technology passes below the inguinal ligament. It then bifurcates into the PFA and SFA. An anatomical knowledge of the level of origin for the PFA is of import in avoiding retroperitoneal bleeding, iatrogenic femoral arterial-venous fistula and/or formation of a pseudo aneurysm. The most lethal complication of femoral access remains retroperitoneal hemorrhaging due to a high puncture. Thus, the best offset pace toward reducing the incidence of retroperitoneal bleeding is to foreclose high punctures.
3.2.1. Training of an antegrade puncture
Every bit nosotros perform in a retrograde puncture, two kinds of wires should be at hand. The initial sheath we place is always the 4Fr size sheath ( Effigy 32 ).

Figure 32.
Preparation of antegrade puncture. 18G needle, 4Fr sheath and ii kinds of wire at mitt.
The chief reason is, when obtaining access to the CFA fails, the sheath can be easily withdrawn or left in the PFA. In one case placing the sheath in the SFA is successful, it can be changed to any sheath as desired. Pointing to the middle of the femoral head, local xylocaine should be given effectually the inguinal ligamentum ( Figure 33 ).

Figure 33.
Antegrade puncture of common femoral artery. Puncture site. (A) Locate center of femora; head. (B) Local xylocaine to exist given at inguinal ligamentum.
Echo was applied in the same way. Withal, the proximal CFA to external iliac avenue is well observed by repeat and may consequence in a very loftier puncture site. Nether fluoroscopic guidance with repeat help, bespeak to the middle of the CFA.
3.2.ii. Puncture
A puncture should be made by aiming an imaginary line over the center of the femoral head. The maximum level of bifurcation should exist at or beneath the junior border of the femoral head ( Figure 34A ). In virtually 1/4th of cases, bifurcation locates in the CFA ( Figure 34B ). In Figure 34B , the bifurcation signal is in the centre of the CFA and there is a brusque margin for the antegrade puncture site.

Figure 34.
Bifurcation point of CFA to SFA. (A) Level of the bifurcation is below the inferior edge of the femoral caput. (B) The bifurcation signal is in the centre of CFA and but short margin for platonic puncture sire. CFA, common femoral avenue.
3.two.three. Two-wire technique
Even when the puncture site is above the bifurcation, the wire may go to the PFA. In this state of affairs, we use a ii-wire technique ( Effigy 35 ). If the wire goes to the PFA, the first pace is to place a 4Fr sheath into the PFA. Two short 0.025 in. wires are inserted into the RFA ( Figure 35A ). Withdrawing the sheath, one 0.025 in. wire should exist manipulated into the SFA ( Figure 35B ). In one case the SFA is accessed, leaving one wire in the PFA, the other wire should be advanced to the SFA ( Figure 35C ). Later on confirming the wire in the SFA, the other PFA wire is withdrawn and a 4Fr sheath should be placed into the SFA ( Effigy 35D ). If the sheath comes out, it can be repositioned back into the PFA by a 0.025 in. wire.

Figure 35.
Two wire technique. (A) If wire goes to PFA, the offset step is to identify a 4Fr sheath into PFA. Ii short 0.025 in. wires are inserted into PFA. (B) Withdrawing sheath, one 0.025 in. wire to find SFA leaving another wire in PFA (
3.2.4. Loftier bifurcation case
Later on surveying the CFA by echo, we may find loftier bifurcation of the SFA and PFA. In these cases, high puncture carries the risk of retroperitoneal haemorrhage. The puncture point should be in the range of the femoral caput. In this situation, puncturing the SFA is one pick. In Figure 36 , in that location is high bifurcation and a CFA puncture is almost impossible. In this case, we decided to puncture the proximal SFA.

Figure 36.
SFA puncture in loftier bifurcation case. Afterwards surveying CFA by repeat, observed high bifurcation of SFA and PFA. In this state of affairs, puncturing SFA is one option.
3.2.5. Sheath kinking
The angle of puncture should be more than than 60° and almost vertical. After sheath insertion, intendance to avert sheath kinking is brash. Once a hematoma is observed with sheath kinking, change to a larger size anti-kink sheath is necessary. In Effigy 37 , the initial 4Fr sheath was kinked ( Figure 37A ) and hematoma formation was detected. After the 4Fr sheath was replaced with a 6Fr sheath, the hematoma was stabilized ( Figure 37B ).

Figure 37.
Sheath kinking during antegrade puncture. (A) Angle of puncture is more than than 60° and about vertical. After inserting sheath, observed sheath kinking. (B) 4Fr sheath replaced with 6Fr sheath and hematoma stabilized.
Advert
4. CO2 angiography
The number of patients with chronic kidney disease (CKD) complicated with PAD is significantly increasing. In these patients, iodinated contrast may enhance the risk of contrast-induced nephropathy (CIN). CIN is an acute renal injury and may lead to irreversible loss of renal function. Carbon dioxide (CO2) gas angiography is indicated for those with renal insufficiency and loftier-risk patients who are allergic to iodinated contrast fabric [7]. CO2 is imaged using digital subtraction equipment with a COtwo software program. Modernistic DSA equipment has a software programme that allows integration of multiple images into a single blended image.
iv.1. CO2 delivery organisation
The arrangement consists of a medical form CO2 gas cylinder with a regulator, a disposable sterile plastic tube with a bacteria-removal filter, and a 50-ml delivery syringe ( Figure 38 ).

Figure 38.
CO2 delivery system. (A) A disposable, sterile plastic tube with a leaner-removal filter and a 50-ml delivery syringe; (B) a medical grade CO2 gas cylinder with a regulator.
Drove of CO2 to the syringe and injection arrangement should be separated to avoid erroneous gas injection to an artery ( Figure 39 ).

Figure 39.
Split organization between CO2 suction and injection. Collection of CO2 to syringe (left) and injection system (right) should exist separated to avoid erroneous gas injection to artery.
The gas should be purged three to four times during collection to prevent room air contamination from the tube and commitment syringe in the circuit and and then filled with gas at a stationary menstruum of 2 l/min. Almost twoscore cc of aspirated gas was filled into the delivery syringe and xxx–40 cm3 of CO2 gas was manually injected into the vessel leaving about 5 cmthree in the injection syringe ( Figure 40 ).

Effigy 40.
Infusion and injection of CO2 gas past 50 cc syringe. (A) Gas was purged 3–four times during collection to exclude room air contamination from the tube and delivery syringe in circuit. Filled with gas at a stationary flow of 2 50/min. (B) 40 cc of aspirated gas filled into delivery syringe, 30–40 cm3 of CO2 gas manually injected into the vessel, leaving about 5 cm3 in the injection syringe.
Afterward gas injection, the remaining gas and blood were carefully aspirated into the syringe. Gas injections were spaced at least xxx s apart. Although we exercise not have feel in mechanical injection, transmission injection is sufficient to inject 30–40 cc of COtwo. Nonetheless, the safe of injecting large amounts of CO2 is not guaranteed [8]. If a patient complains of abdominal pain, further CO2 injection should exist avoided. And if the angiogram shows a slow flow, further CO2 commitment by syringe should also exist stopped ( Table 3 ).
| - Make a dissever organization with CO2 cylinder - Average dose of CO2 is well-nigh 30 ml by using 50 ml syringe - Exist sure complete air excretion - Manual injection not a mechanical injection - If patient complains of intestinal pain, avert further injection - When a wearisome flow are observed, avoid an further CO2 injection |
Table 3.
Setup of COii commitment system and COii injection.
4.2. Iliac avenue angiography by CO2
The iliac avenue is a large sized vessel and its arrival is the larger abdominal aorta. Moreover, there are 2 internal iliac arteries and 2 femoral arteries. CO2 angiography requires displacement of all or most of the blood to achieve adequate images. Due to such anatomical reasons, the iliac artery is not well suited for CO2 angiography. In Figure 41A , CO2 was administered from a 5Fr pigtail catheter at the final aorta and, in the left external iliac artery, CO2 was unfilled and in that location appears to be stenosis. With dissimilarity angiography, no stenotic lesion is seen in the left external iliac avenue ( Figure 41B ).

Figure 41.
Iliac artery angiography by CO2 and DSA. (A) COtwo administered from 5Fr pigtail catheter at the final aorta, in left external iliac artery, COtwo unfilled and stenosis suspected. (B) In DSA, no stenotic lesion in left external iliac avenue observed.
In Figure 42A , total occlusion of the left external iliac artery is observed. CO2 injection from the terminal aorta shows chronic full apoplexy (CTO) of the left external iliac avenue. To ostend CTO, a crossover sheath was positioned at the left common iliac artery and CO2 injection was repeated at a right anterior oblique (RAO) projection of 30° ( Figure 42B ). In this angiogram, CTO is clearly visualized and the left mutual femoral artery is well observed via the collateral catamenia from the deep circumflex artery. In CO2 angiography of the iliac artery, the angiogram is hampered by bowel and gas movements.

Figure 42.
Left external iliac artery occlusion past CO2 angiography. (A) CO2 injection from last aorta in AP view. Total occlusion of left external iliac artery. (B) CO2 injection from left common iliac artery by RAO thirty.
In Figure 43A , the left iliac artery is not seen, but dissimilarity angiography shows a clear motion-picture show of the entire iliac arteries ( Figure 43B ).

Effigy 43.
Bowel gas in iliac avenue angiography. (A) In CO2 angiography, left external iliac artery is hampered past bowel gas. (B) DSA shows a articulate pic of whole iliac arteries.
Generally speaking, when the iliac artery is non well visualized by CO2 angiography, increasing the volume of CO2 in the iliac abdominal aorta might be considered. However, there are important visceral vessels and the gamble of diverse complications due to the injection of gas in these vessels must also exist considered.
4.3. Femoropopliteal avenue angiography past CO2
Visualization by CO2 angiography is all-time suited for the femoropopliteal artery segment. The main reason is that the superficial femoral artery (SFA) is a straight vessel with small branches. The vessels sizes are most four–vii mm and could hands be filled by CO2 gas. In Figure 44 , in that location are three kinds of SFA angiograms for the aforementioned patient. Digital angiography enabled visualization of the groundwork ( Effigy 44A ) while DSA could obtain the highest quality angiogram ( Figure 44B ). CO2 angiography has poor visibility of small distal branches. Withal, it could visualize SFA fairly well and can be used as a substitute for contrast angiography ( Figure 44C ).

Effigy 44.
SFA angiography past digital, DSA and CO2. (A) Digital angiography could visualize background to be used every bit reference. (B) DSA obtained most accurate epitome. (C) CO2 angiography cannot replace digital angiography, just can be used as a substitute for dissimilarity angiography.
Similarly, the popliteal artery could be well observed even with the COii angiogram ( Figure 45 ).

Figure 45.
Popliteal artery angiography by digital, DSA and COtwo. (A) Digital angiography could visualize background to be used as reference. (B) DSA obtained nigh accurate image. (C) CO2 angiography obtained like paradigm to DSA.
In the DA angiogram, distal SFA is not well visualized compared to the DSA image ( Effigy 45A ). In fact, a perfect paradigm was obtained by DSA ( Figure 45B ). The COtwo angiogram shows a fairly clear picture of the distal SFA and popliteal avenue ( Figure 45C ) while the correct femoropopliteal artery was visualized by CO2 ( Figure 46 ). In proximal SFA, separation betwixt the SFA and deep femoral artery (DFA) is well observed ( Effigy 46A ). In the mid-SFA, no stenosis is seen ( Effigy 46B ). In the distal SFA and popliteal artery, moderate stenosis is detected ( Figure 46C ).

Figure 46.
CO2 angiography for right femoropopliteal avenue. (A) In proximal SFA, separation betwixt SFA and deep femoral avenue (DFA) is well shown by RAO view. (B) In mid-SFA, there is no stenosis. (C) In distal SFA and the popliteal artery, there is moderate stenosis.
Comparisons between COii angiography and digital angiography for the diseased SFA are shown in Figures 47 and 48 . Stenosis is seen in the distal SFA in Effigy 47 . Both COtwo ( Figure 47A ) and DA images ( Figure 47B ) could place distal SFA stenosis.

Figure 48.
Left SFA occlusion by COtwo and DSA. (A) CO2 angiography shows totally occluded left SFA. (B) DSA shows clearer epitome with more collateral visualization.

Figure 47.
Left SFA stenosis past CO2 and digital angiography. (A) COtwo angiography shows moderate stenosis in mid SFA and tight stenosis in distal SFA. (B) Digital angiography confirmed these ii lesions. Image quality is similar between COtwo and digital angiography.
The totally occluded left SFA was well visualized past CO2 angiography ( Effigy 48A ). Although DSA shows a clearer image with a rich collateral network ( Figure 48B ), the CO2 image tin can also be used for interventional work.
The CTO of the left SFA was intervened using CO2 angiography ( Effigy 49 ) in a patient with stage 4 CKD. The COtwo angiogram showed typical CTO of the SFA ( Effigy 49A ). After successful wiring, balloon angioplasty was performed ( Figure 49B ). Dissimilarity was but used in the terminal angiogram ( Effigy 49C ).

Figure 49.
Left SFA CTO intervention past COtwo angiography. (A) COii angiography showed typical SFA CTO. (B) After wiring, balloon angioplasty was performed. (C) Contrast used only in terminal angiogram.
four.4. Below the articulatio genus angiography past CO2
CO2 angiography cannot be applied in below the knee (BK) work. The arterial vessel size beneath the knee is between 1.5 and iii mm in diameter and the accurateness of COtwo angiography is bereft. In a higher place all, in BK cases, near of the patients have critical limb ischemia and cannot tolerate large amounts of gas injection. In Figure fifty , proximal right below the genu angiography was performed past COii ( Figure 50A ) and DSA ( Figure 50B ). In the CO2 angiogram, stenosis of the peroneal trunk could be seen; however, the right anterior tibial artery and posterior tibial avenue are not well visualized when compared to DSA.

Figure 50.
Below the knee angiography by COii and DSA. (A) Proximal below the arteries past CO2 angiography. Stenosis of peroneal body could exist seen, otherwise, unable to identify right anterotibial and posterior tibial artery. (B) DSA shows detail of proximal below the knee arteries with minor branches.
4.5. Problems of CO2 angiography
CO2 angiography can be performed with minimal or no dissimilarity media and tin can exist used on CKD patients. However, CO2 angiography carries several potential risks (8). Gas delivery into the vessel is basically contraindicated. Moreover, erroneous injection of excessive volumes may upshot in catastrophic clinical consequences. There are many reports about transient lower limb pain and transient intestinal pain. Fujihara et al. have conducted a multi-center prospective COtwo study and accept reported that two patients (2%) developed COtwo-related non-occlusive mesenteric ischemia which resulted in death. These not-occlusive mesenteric ischemia cases were acquired by the trapping of CO2 gas in the celiac, superior and/or junior mesenteric arteries [8]. The quality of CO2 angiography is still not articulate enough in the iliac artery and should not be employed in below the knee arteries. It should be used for the femoropopliteal avenue although, even in the femoropopliteal artery, precise lesion evaluation may be difficult in some cases. Other complementary modalities such as surface echo, IVUs and/or pressure measuring should also be employed to confirm lesion severity ( Table 4 ).
| - Cause abdominal hurting and leg pain - Poor quality angiogram in iliac artery by bowel gas and movement - Non applicable to below knee artery - Rapid transition to venous apportionment - Risks of cognitive infarction - Risk of nonobstructive mesenteric ischemia |
Table 4.
Issues of CO2 angiography.
In treating claudication, safety is the offset priority and so that CO2 use may exist limited for virtually patients except those who have anaphylaxis to iodinated contrast media.
References
- 1.
Grynne BH, Nossen JO, Bolstad B, Borch KW. Main results of the first comparative clinical studies on Visipaque. Acta Radiol Suppl. 1995;399:265–70. - 2.
Nicola R, Shaqdan KW, Aran Grand, Mansouri M, Singh A, Abujudeh HH. Contrast-induced nephropathy: identifying the risks, choosing the right agent, and reviewing constructive prevention and management methods. Curr Probl Diagn Radiol. 2015;44(half-dozen):501–4. - 3.
Gedikoglu M, Oguzkurt L, Gur Southward, Andic C, Sariturk U, Ozkan C. Comparison of ultrasound guidance with the traditional palpation and fluoroscopy method for the common femoral artery puncture. Catheter Cardiovasc Interv. 2013;82(vii):1187–92 - 4.
Lo RC, Fokkema MT, Curran T, Darling J, Hamdan Advertisement, Wyers M, Martin K, Schermerhorn ML. Routine use of ultrasound-guided admission reduces access site-related complications after lower extremity percutaneous revascularization. J Vasc Surg. 2015;61(2):405–12. - five.
Fujihara M, Haramitsu Y, Ohshimo K, Yazu Y, Izumi E, Higashimori A, Yokoi Y. Advisable hemostasis by routine use of ultrasound repeat-guided transfemoral access and vascular closure devices after lower extremity percutaneous revascularization. Cardiovasc Interv Ther. 2016. [Epub alee of print] PubMed PMID: 27430637. - 6.
Cho KJ. Carbon dioxide angiography: scientific principles and do. Vasc Specialist Int. 2015;31(three):67–80. - 7.
Kawasaki D, Fujii K, Fukunaga Chiliad, Masutani Yard, Nakata A, Masuyama T. Condom and efficacy of endovascular therapy with a simple homemade carbon dioxide commitment system in patients with ileofemoral artery diseases. Circ J. 2012;76(seven):1722–8. - 8.
Fujihara Yard, Kawasaki D, Shintani Y, Fukunaga M, Nakama T, Koshida R, Higashimori A, Yokoi Y. Endovascular therapy past CO2 angiography to preclude contrast-induced nephropathy in patients with chronic kidney disease: a prospective multicenter trial of COii angiography registry. Catheter Cardiovasc Interv. 2015;85:870–7.
Submitted: September seventh, 2016 Reviewed: December 7th, 2016 Published: March 22nd, 2017
© 2022 The Writer(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 License, which permits use, distribution and reproduction for non-commercial purposes, provided the original is properly cited.
Source: https://www.intechopen.com/chapters/53791
0 Response to "How to Know if You Popped an Artery"
Post a Comment